Photoelectric Effect
The emission of electrons, by metal surface when light falls on them, is known as photoelectric emission and the phenomenon is known as photoelectric effect.
How was the phenomenon of photo-electricity discovered?
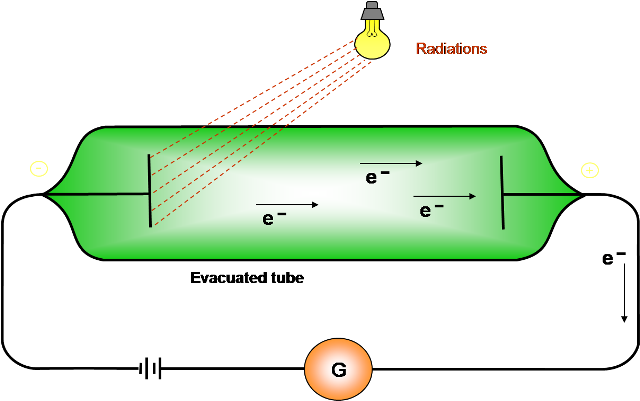
The initial discovery of the phenomenon of photo electricity can be said to have been made by Hertz. He observed that the spark. across the gap of his ‘detector, passed much more easily when ultraviolet light was allowed to fall on it. The phenomenon was later on formally discovered by Hallwachs. He discovered that when (suitable) light is allowed to fall on the cathode of an evacuated tube, current flows towards the anode. No current would flow if the anode was irradiated with light. This indicated that negatively charged particles can get emitted from the cathode when it is irradiated with (suitable) light.
Explain how it was established that photo particles are negatively charged electrons?
The identity of the photo particles with electrons was suspected when it was observed that the photo particles are also negatively charged (like the electrons). The exact identity was established when Lenard, through his detailed quantitative measurements, showed that the (e/m) of the photo particles was the same as that of electrons. All the photo particles Irrespective of the nature of the metal of the cathode or the frequency of the irradiating light always had the same value of elm as those of the electrons.
Light from a bulb is falling on a wooden table but no photoelectrons are emitted, Why?
Wood is a bad conductor (insulator) and hence, contains no free electrons.
Why is alkali metals most suited as photo- sensitive materials?
The work function for these metals, being very low, even lights of low frequency can cause photo-emission from them.
Do all the photoelectrons emitted from a metal surface possess the same energy?
No, there is an energy distribution amongst the emitted photoelectrons, with their energy ranging from (zero to a certain maximum value = h (v - vo) characteristic of the frequency of the incident light.)
Does the maximum energy with which electrons is emitted from a metal surface depend
upon intensity or frequency of the incident light?
The maximum energy of the emitted photoelectrons depends on the frequency but not on the intensity of the incident light.
Is photoelectric emission possible at all frequencies? Give reasons for your answer.
No: photoemission is possible only for a certain minimum frequency value (called the threshold frequency, represented by 'vo' ). This is because the incident photons must have at least the minimum energy equivalent to the work function W0 of the metal.
Why photoelectric effect cannot be explained on the basis of wave theory of light?
Electro-Magnetic Waves theory’s failure vis-à-vis Photoelectric effect;
The Electro-Magnetic Waves theory could not explain the experimental results on photoelectricity. This is because as per the Electro-Magnetic Waves theory, it is the forces, exerted by the electric field of the incident light beam, that bring about an increase in the energy of the free electrons present in the metal. However the, above mechanism of gain of energy by the free electrons from the incident light beam, does not fit in with the observed features of photoelectricity This can be seen as follows:
1) The energy of the incident radiation - as per the Electro-Magnetic Waves theory must be regarded as smoothly and evenly distributed over its associated wavefront. Hence, each electron would intercept only an insignificantly small amount of energy and would require a finite time (hours, days or weeks) to accumulate enough energy to escape from the metal. Experimentally, however, we observe that electron ejection commences without (any noticeable) delay.
2. The classical wave theory was unable to account for the existence of the threshold frequency. There should be no reason why sufficiently intense beam of low frequency radiation would not be able to produce photoelectricity, if low intensity radiation of higher frequency could produce it.
3. On the classical theory, we expect that light of high intensity, i.e.. consisting of strong fields.
would give higher kinetic energy to the liberated electrons. This, however, does not happen. Also the electron’s energy should not depend particularly on the light frequency. But it does. It is obvious: therefore, that the photoelectric effect cannot be explained on the basis of the classical Electro-Magnetic Waves theory of light.



No comments:
Post a Comment